
Overview-
The Industrial Revolution has undeniably brought the world closer by boosting trade volumes among countries. With the primary desire to expand their markets, businesses have started exchanging goods at an unprecedented rate.
On the contrary, International trade exposes consumers to goods that are unavailable in their own countries, or that are more expensive domestically. This is because international trade has made the market more competitive, giving consumers access to a wider variety of products from multiple sources, ultimately offering cheaper products.
However, when it comes to transporting consumable products, it becomes the responsibility of both countries that the products, that are being exported and imported, are safe to consume.
This is particularly crucial in the pharmaceutical manufacturing industry, where products directly impact human health. Acting promptly on this, regulatory authorities like the FDA and the EU established some regulations and guidelines governing the production, packaging, and labelling of medicines to prevent contamination. Since then, meeting certain regulatory standards has become a mandate for pharma manufacturers across the world.
The UK Pharma Manufacturing Market-
The UK manufacturing market is one of the largest pharma producers in the world, acting as the major contributor to the UK’s economy. The UK pharmaceutical industry is not limited to supplying medicines domestically but serves other markets as well internationally.
The major portion of the country’s revenue comes from exports, as UK-manufactured drugs always remain in high demand worldwide.
However, despite having a strong pharmaceutical manufacturing base, UK-based pharma manufacturers often struggle to consistently meet the complex regulatory requirements. This non-compliance leads to trade restrictions, significant revenue loss, and legal penalties as well.
But, to stay competitive in the global world, UK-based pharma manufacturers need to comply with the regulatory standards, avoiding such costly setbacks.
But, what these manufacturers should adopt to get themselves to navigate this ocean of compliance easily without compromising on the efficiency of other supply chain operations.
ERP software for pharma manufacturing is a comprehensive solution capable of helping manufacturers stay compliant throughout their manufacturing journey. But before getting into the details of how an ERP for the Pharma Industry does this, let’s understand the fundamentals of how the FDA and the EU work.
Decoding FDA & EU-
Both the FDA and the EU regulations work toward the same goal of ensuring products are safe and compliant. Both portray current acceptable production practices with detailed guidance on the principles of regulations. However, they differ in procedures and requirements, let’s have a quick look.
FDA (US):
- This regulation involves following current good manufacturing practices, focusing on controlling the manufacturing, processing, and storage of drugs, requiring meticulous documentation and validation.
- Moreover, this regulation even oversees drug approval, manufacturing, and labelling intensively within the United States.
- It even requires thorough documentation and electronic records, including batch records, electronic signatures, and audit trails.
EU:
- This regulation undertakes European Good Manufacturing Practices, which are similar to the FDA guidelines but include additional requirements like strict focus on batch quality control.
- It even involves greater emphasis on detailed traceability requirements from raw materials to the final product.
Health hygiene and clothing of personnel are also addressed, and mandating the drug manufacturer to maintain the records of the same with the information on name, address, qualification, and the type of service that has been performed by them.
Reiterating what has been mentioned earlier in the blog- to ensure that all the mandates are being followed responsibly throughout the manufacturing process right from procuring drugs to processing and ultimately delivering medicines to respective customers, a robust ERP system for Pharmaceuticals is the need of the hour.
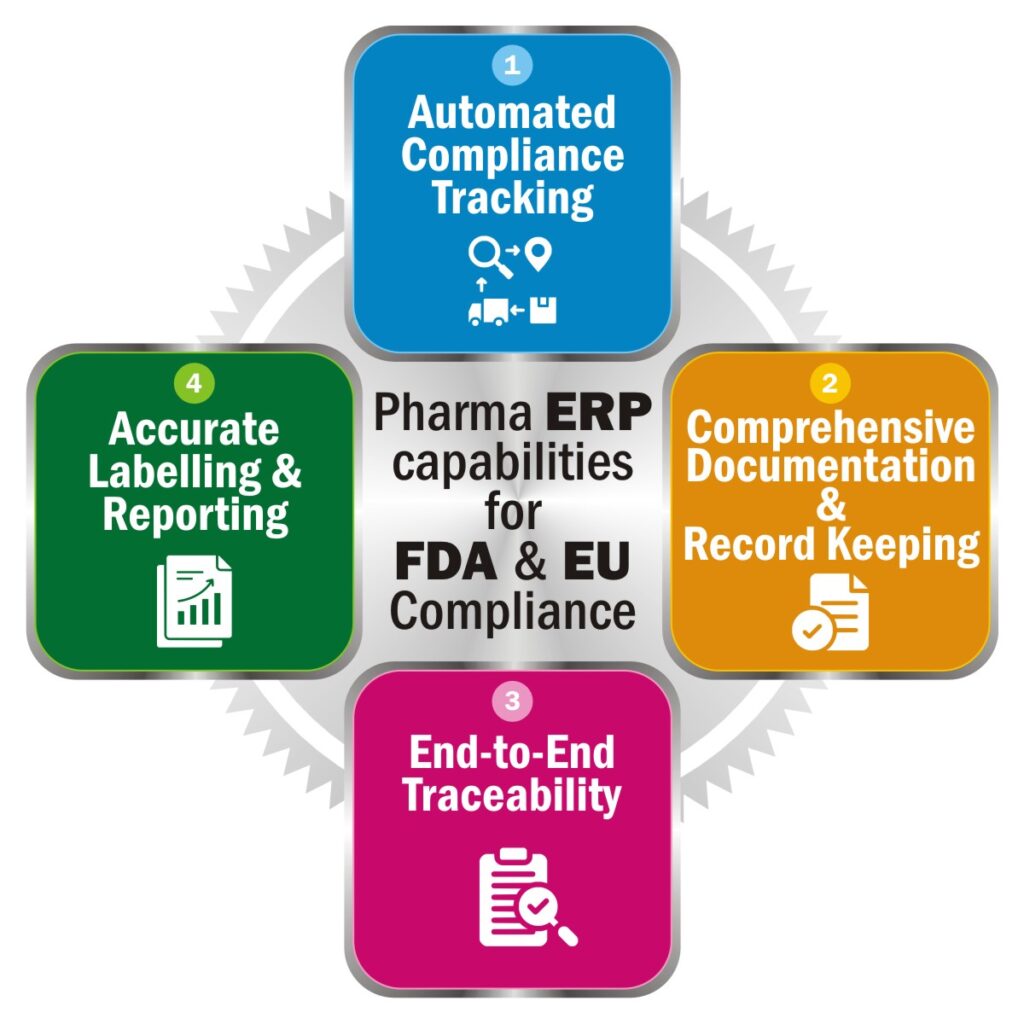
Yes, a competent ERP for Pharma performs this task seamlessly for you along with digitizing, managing and streamlining all business critical operations like Formulation & R&D, Planning & Production, Inventory & Lot Management, Finance & Accounting, and Quality & Compliance. This blog focuses on how an ERP solution helps meet FDA & EU Regulations. Have a read-
1. Automated Compliance Tracking-
ERP software offers a built-in compliance framework incorporating FDA & EU standards related to cGMP, storage of drugs, etc. Its real-time monitoring feature even generates prompt alerts in case of compliance gaps, ensuring that processes align with the guidelines always.
2. Comprehensive Documentation & Record Keeping-
Meticulous documentation is crucial in the pharma industry to ease regulatory audits. ERP system offers a centralized digital repository for all critical documents, including drug handling and storage protocols, manufacturing processes, batch records, packaging and labelling details, electronic signatures, and more. This ensures secure access, efficient retrieval, and enhanced compliance management.
3. End-to-End Traceability-
Complete traceability is the common requirement among both the FDA & the EU regulation, and the ERP software performs this task with utmost precision & accuracy, ensuring every movement is tracked.
Raw Materials Traceability-
Capturing details about raw materials including batch numbers, quality certifications, and supplier information, ERP solution ensures only approved materials are used in production.
Moreover, by tagging allergen-specific ingredients and maintaining segregated records, ERP helps avoid cross-contamination during production.
Production Process Traceability-
Pharma ERP software meticulously tracks each stage of the manufacturing process, capturing comprehensive batch records that include process parameters, operator details, equipment usage, and environmental conditions.
This level of detailed documentation ensures that compliance is upheld throughout production, minimizing risks and maintaining regulatory standards at every step. Performing real-time data logging of production processes that facilitates identifying deviations quickly to enable prompt corrective actions.
Batch Tracking-
Allotting a unique number, an ERP system maintains the entire history of every batch including information on ingredients, process conditions, quality tests, packaging requirements, etc. In case of quality or safety issues, this functionality helps manufacturers identify the specific defective batch, facilitating swift recall.
Quality Inspections & Testing Records-
At required points in the production cycle, ERP software facilitates establishing quality control checks along with storing test results and approval statuses, certifying that only “QC pass” batches are released.
Accurate Labelling & Reporting-
Incorporating all the required information such as batch number, expiration dates, allergen warnings, and active ingredient details, Pharmaceutical ERP ensures that each product is labelled accurately.
This enables clear identification of products throughout the supply chain and ensures compliance with FDA & EU regulations.
Moreover, the FDA mandates MBRs to ensure consistency and adherence to pre-approved manufacturing processes as outlined in 21 CFR Part 211.186. While, the FDA requires BMRs for batch traceability and to verify compliance with GMP as outlined in 21 CFR Part 211.188.
Both these –MBR as well as BMR can be generated by an ERP software in clicks ensuring compliance required by the FDA (U.S. Food and Drug Administration) as part of good manufacturing practices (GMP).
Closing Thoughts-
By offering this improved level of control over the supply chain operations, an ERP helps manufacturers fulfil all the demands related to FDA & EU regulations, preventing them from getting caught in costly legal issues and fines.
Being a pharma manufacturer, if you are looking for such a comprehensive and competent ERP solution then, BatchMaster ERP for Pharma is the ideal technological partner for you. Get in touch with our team of experts to know more about it.





