Pharmaceutical ERP Software for UK Manufacturers
Industry-Leading BatchMaster ERP for Pharma Companies in UK – Streamline Processes, Ensure Quality, and Drive Growth.

Simplify UK Pharmaceutical Manufacturing with ERP Software Tailored to Your Business
The UK pharmaceutical industry is one of the most tightly regulated and competitive sectors, with strict regulatory compliance, GMP requirements, and growing pressure for faster time-to-market, cost control, and supply chain transparency.
Rising material costs, evolving quality standards, and global competition further add to these challenges. To overcome them, companies need an integrated Pharma ERP software that streamlines formulation, production, inventory, quality, and compliance—all in one system.
That’s why leading pharma businesses across the UK and Europe trust BatchMaster’s Pharmaceutical ERP system — the leading ERP for pharma industry, to simplify complexity, ensure compliance, and drive growth—whether you’re a contract manufacturer, generics producer, or API and life sciences company.
Schedule a Demo of BatchMaster Pharma ERP Software
schedule-a-discovery-call
Key ERP Modules for the UK Pharmaceutical Manufacturing Industry
Trusted by Leading Pharmaceutical Manufacturers
From global enterprises to local innovators, BatchMaster’s pharmaceutical ERP helps manufacturers streamline operations, support regulatory and GMP-compliance, and enable sustainable growth.
Transform Your Pharma Manufacturing Business with BatchMaster ERP
Unlock Predictive Insights, Drive Success, and Achieve Amazing Results!
Key Features of Pharmaceutical Manufacturing Software
Create, scale, and modify formulas with precision and complete version control. This module helps maintain consistency, track changes for audits, and supports by-products and co-products — essential for pharmaceutical production.
Our pharmaceutical ERP software helps companies maintain detailed specifications for every raw material and product to meet GMP and regulatory standards.
Meet UK, EU, and international regulations with electronic signatures, secure access controls, and detailed audit trails — tracking every stage from material issue to batch release.
eBMR replaces paper-based records with a secure digital system, providing real-time production tracking and complete traceability. It ensures accuracy, compliance, and faster batch reviews, keeping manufacturers fully audit-ready from start to finish.
Generate accurate COAs automatically for quicker product release and regulatory approval.
Quickly trace any lot from raw material to finished product, enabling fast, precise recalls when needed with our advance lot traceability software.
Manage third-party manufacturing operations, track outsourced processes, and maintain visibility over quality and timelines.
Create accurate production plans to meet demand, optimise resources, and reduce bottlenecks. With MRP module, ensure timely material availability, improve inventory control, minimise downtime, and reduce carrying costs for a more efficient and cost-effective operation.
Accelerate product innovation with tools to manage formulation, costing, trials, and compliance documentation. NPD helps UK manufacturers reduce time-to-market while staying aligned with FDA regulations.
ERP Delivering Success Across Pharmaceutical Sectors
Biotechnology
Vaccines

Dietary

API

Veterinary

Nutraceuticals
Why Pharma Manufacturers Trust BatchMaster – Leading Pharma ERP Software for the UK & Europe
With BatchMaster software solutions, your company can be compliant with today’s ever more stringent FDA regulatory mandates, as well as follow current good manufacturing practices (cGMP). Provide auditors with audit trials of key transactions, which include electronic signatures (CFR 21 11) and key reports, such as the Master Batch Record (MBR) and Batch Manufacturing Record (BMR). In addition, BatchMaster Software can assist companies achieve GMP Validation of their processes.
BatchMaster ERP for pharma integrates seamlessly with leading financial systems like SAP Business One , Sage 50/100/200/300, QuickBooks, and Xero, offering a reliable upgrade to your financial and manufacturing capabilities. BatchMaster ERP serves the entire pharmaceutical value chain — from APIs and pharmaceutical intermediates to finished formulations like tablets, syrups, and injectables.
By choosing BatchMaster, pharma manufacturers gain scalability, reliability, and real-time visibility, ensuring they stay competitive in today’s fast-paced and regulated market.
Business Benefits of ERP Software for Pharma Industry
- Enhanced Quality, Consistency, and Speed to Market: Improves drug delivery efficiency, ensuring high-quality and consistent products reach the market more quicker.
- Faster Introduction of New Products at Competitive Prices: Facilitates swift market entry for new products at competitive prices, helping manufacturers outpace competitors.
- Waste Reduction and Optimal Inventory Management: Reduces wastage, optimises material utilisation, and maintains inventory balance, thereby controlling overhead costs.
- Compliance with Stringent Regulations: Adheres to strict industry rules and regulations, ensuring compliance throughout the entire manufacturing process.
- Risk Mitigation through Quality Delivery: Minimises risks by consistently delivering superior-quality products at the right time and price.
- Swift Preventive and Corrective Actions: Enables a quick response to implement preventive and corrective actions, ensuring product integrity.
- Responsiveness to Customer Demands: Enhances the ability to respond promptly to changing customer requirements.
- Improved Operational Efficiency and Profitability: Streamlines operational workflows, contributing to higher profits.
- Real-time Batch Monitoring for Informed Decisions: Provides accurate, real-time batch monitoring data for making informed business decisions.
- Formula Secrecy Maintenance: Safeguards formula confidentiality and intellectual property.

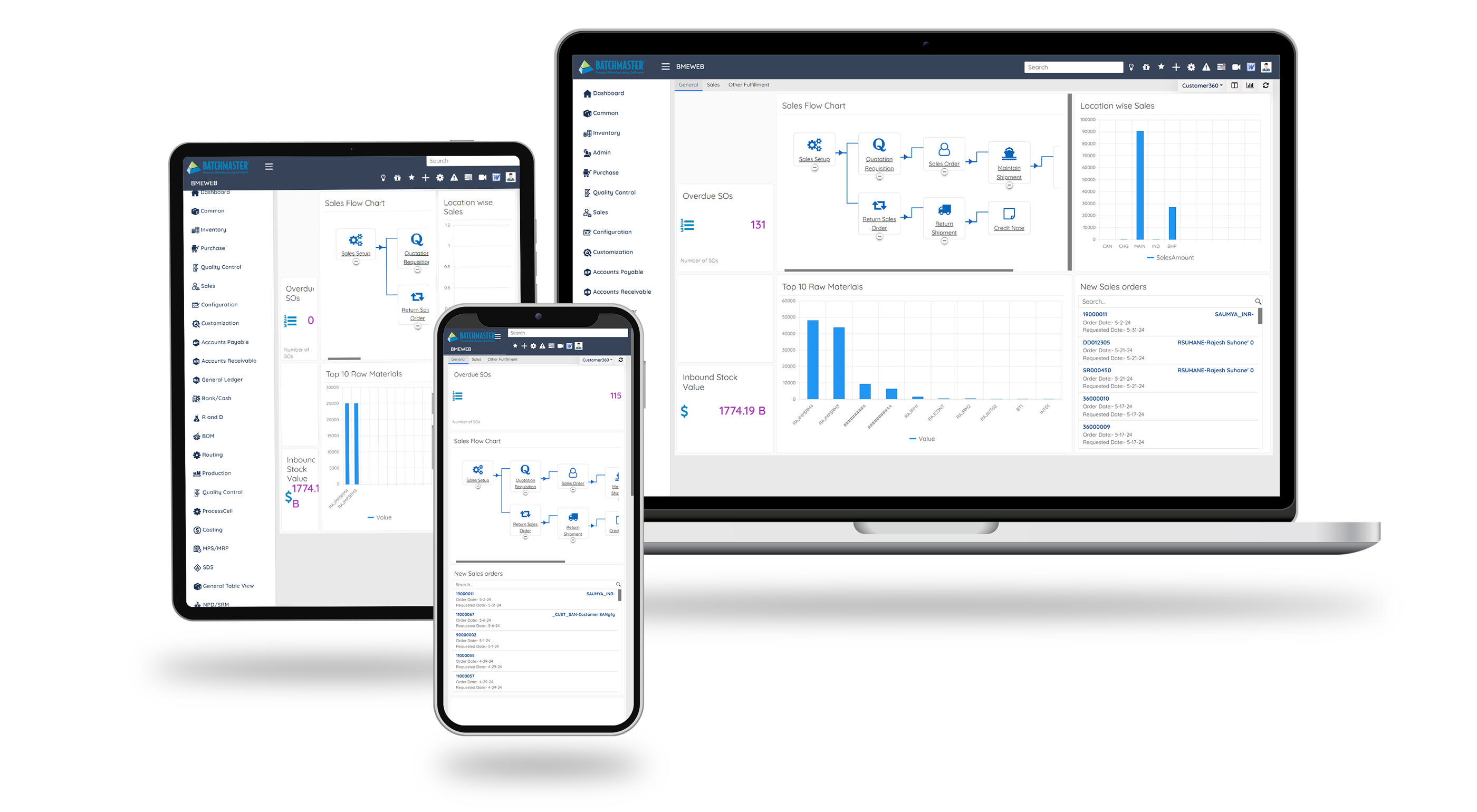
See BatchMaster ERP in Action – Book Your Free Demo Now
See how BatchMaster ERP streamlines compliance, enhances workflows, and drives faster, smarter food operations.
Frequently Asked Questions
Yes, it’s designed for businesses of all sizes. With cloud and on-premise options, it scales as your operations grow, making it ideal for SMEs as well as large pharmaceutical enterprises.
Look for modules like, formulation management, lot/batch tracking, electronic batch/ master batch records, quality assurance, QC workflows, compliance reporting, and integration with lab/QA systems
BatchMaster ERP is purpose-built for pharmaceutical manufacturing, offering built-in compliance with EU GMP, and FDA 21 CFR Part 11 etc. Unlike generic ERPs, it delivers industry-specific features, seamless integration with systems like Sage and SAP Business One, and scalability for both SMEs and large enterprises.
We provide comprehensive training, onboarding, documentation, and ongoing UK/EU-based support. Regular updates, guidance, and customer success services help ensure stable operations and compliance.
Looking To Find the Best Solution for Your Business?
Allow our expert team of solution consultants to review your business operations so that they can offer you the best-possible solution, either on premise or in the cloud, to meet all your industry-specific needs

About Us
BatchMaster Software is one of the market leaders in offering enterprise software solutions for the process manufacturing industries. With in-depth market insights gained through extensive industry experience, including over 3,000 implementations worldwide, we clearly understand the unique challenges of this sector. BatchMaster Software Pvt. Ltd. offers ERP solutions tailored to industry-specific operations and micro-vertical needs. Process manufacturing companies around the globe rely on BatchMaster® to manage nearly every aspect of their manufacturing, distribution, finance and accounting, quality control, and compliance-related operations. Headquartered in Irvine, California, BatchMaster has offices in New Jersey, India, and Mexico.















